NCERT Exemplar Class 10 Science Chapter 2 Acids, Bases And Salts
NCERT Exemplar Class 10 Science Chapter 2 Acids, Bases And Salts are part of NCERT Exemplar Class 10 Science. Here we have given NCERT Exemplar Class 10 Science Chapter 2 Acids, Bases And Salts.
NCERT Exemplar Class 10 Science Chapter 2 Acids, Bases And Salts
Short Answer Type Questions
1.Match the acids given in Column (A) with their correct source given in Column (B).

Ans.(a) —> (iv) (b) —> (iii) (c) —> (ii) (d) —> (i)
Lactic acid is present in curd, acetic acid is present in vinegar, citric acid is present in lemon and oxalic acid is present in tomato.
2.What will be the action of the following substances on litmus paper?
Dry.HCl gas, Moistened NH3 gas, Lemon juice, Carbonated soft drink, Curd, Soap solution.
Ans.Dry HC1 gas will not have any effect on litmus paper. Moistened NH3 gas will turn red litmus blue. Curd, lemon juice, carbonated soft drink will turn blue litmus red. Soap solution will turn red litmus blue.
3.Name the acid present in ant sting and give its chemical formula. Also give the common method to get relief from the discomfort caused by the ant sting.
Ans. Ant sting contains methanoic acid (formic acid). Its chemical formula is HCOOH. The common method to get relief is to apply paste of
4.What happens when nitric acid is added to egg-shell?
Ans. Egg-shell is made up of calcium carbonate which will react with HNO3 to form
5.A student prepared solutions of (i) an acid and (ii) a base in two separate
beakers. She forgot to label the solutions and litmus paper is not available in the laboratory. Since, both the solutions are colourless, how will she distinguish between the two?
Ans.Add phenolphthalein to a portion of each solution in separate test tube. If it turns pink, the beaker contains base whereas if it remains colourless, it is an acid.
If phenolphthalein is not available, pH paper can be used. Acid will turn pH paper red, base will turn pH paper blue.
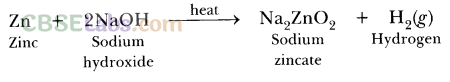
6.How would you distinguish between baking powder and washing soda by heating?
Ans.
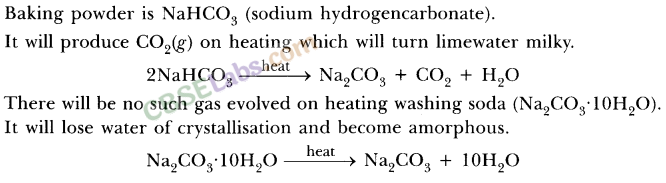
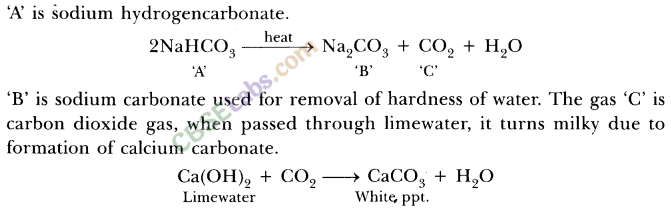
7.Salt A commonly used in bakery products on heating gets converted into another salt B which itself is used for removal of hardness of water and a gas C is evolved. The gas C when passed through lime water, turns it milky. Identify A, B and C.
Ans.
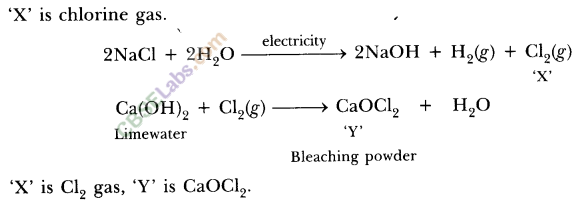
8.In one of the industrial processes used for manufacture of sodium hydroxide, a gas X is formed as by-product. The gas X reacts with lime water to give a compound Y which is used as a bleaching agent in chemical industry. Identify X and Y giving the chemical equation of the reactions involved.
Ans.
9.What are strong and weak acids? In the following list of acids, separate strong acids from weak acids.
Hydrochloric acid, citric acid, acetic acid, nitric acid, formic acid, sulphuric acid.
Ans. Strong acids are those acids which are completely ionised in aqueous solution. Weak acids are those which do not ionise completely in aqueous solution. Strong adds: HC1,
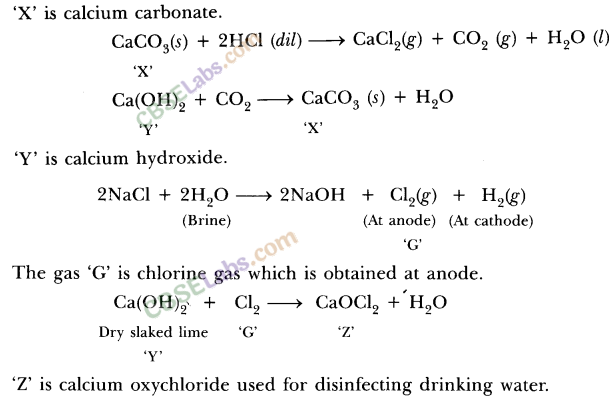
1O.A metal carbonate X on reacting with an acid gives a gas which when passed through a solution Y gives the carbonate back. On the other hand, a gas G that is obtained at anode during electrolysis of brine is passed on dry Y, it gives a compound Z, used for disinfecting drinking water. Identify X, Y, G and Z.
Ans.
11.A dry pellet of a common base B, when kept in open absorbs moisture and turns sticky. The compound is also a by-product of chlor-alkali process. Identify B. What type of reaction occurs when B is treated with an acidic oxide? Write a balanced chemical equation for one such solution.
Ans.‘B’ is NaOH. It absorbs moisture from atmosphere because it is hygroscopic in nature. It is obtained by product of chlor-alkali process.
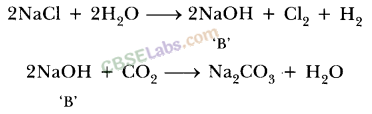
‘B’ reacts with acidic oxide
12.A sulphate salt of Group 2 element of the Periodic Table is a white, soft substance, which can be moulded into different shapes by making its dough. When this compound is left in open for sometime, it becomes a solid mass and cannot be used for moulding purposes. Identify the sulphate salt. Why does it show such a behaviour? Give the reaction involved.
Ans. Salt is CaS04
moulded into different shapes, as 2 moles of CaS04 share 1 mole of HgO molecule.
When it is left in open, it becomes solid mass CaS042H20 (Gypsum) which cannot be used for moulding purposes as it is hard solid mass.
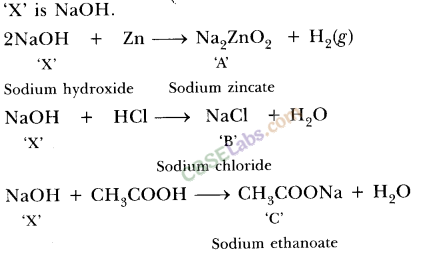
13.Identify the compound X on the basis of the reactions given below. Also, write the name and chemical formulae of A, B and C.
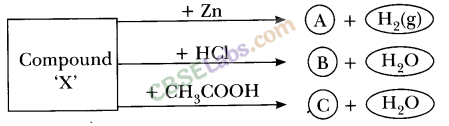
Ans.
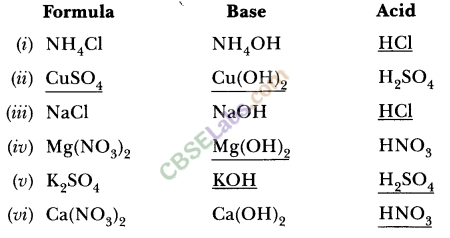
14.Match the important chemicals given in Column (A) with the chemical formulae given in Column (B).

Ans.

15.Fill in the missing data in the following data.
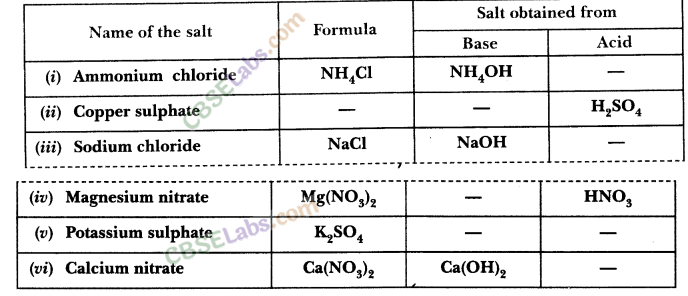
Ans.
16.When Zinc metal is treated with dilute solution of strong acid, a gas is involved which is utilized in hydrogenation of oil. Name the gas involved. Write the chemical equation for the reaction involved and also write a test to detect the gas formed.
Hydrogen gas is liberated
Ans.
Test: Bring a burning splinter near the gas. The gas will burn with ‘Pop’ sound which shows gas is H2.
17.In the following schematic diagram for the preparation of hydrogen gas as shown in the given figure, what would happen if following changes are made ?
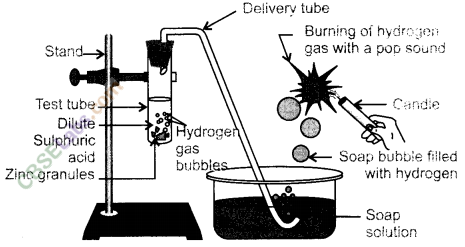
(а)In place of zinc granules, same amount of zinc dust is taken in the test tube.
(b)Instead of dilute sulphuric acid, dilute hydrochloric acid is taken.
(c)In place of zinc, copper turnings are taken.
(d)Sodium hydroxide is taken in place of dilute sulphuric acid and the tube
is heated.
Ans.(a)The reaction will become faster because zinc dust has more surface area.
(b)Nearly same amount of hydrogen gas will be evolved.
(c)No reaction will take place as copper is less reactive than hydrogen.
(d)The reaction will take place and hydrogen gas will be evolved.
18.For making cake, baking powder is taken. If at home your mother uses baking soda instead of baking powder in cake.
(a)How will it affect the taste of the cake and why?
(b)How can baking soda be converted into baking powder?
(c)What is role of tartaric acid added to baking soda?
Ans.(a)The cake will taste bitter due to formation of sodium carbonate.
(b)Tartaric acid should be added to baking soda to convert it into baking powder.
(c)Tartaric acid neutralizes sodium carbonate formed and will not make the taste bitter.
NCERT Exemplar Class 10 Science Solutions
- Chapter 1 Chemical Reactions and Equations
- Chapter 2 Acids Bases and Salts
- Chapter 3 Metals and Non-metals
- Chapter 4 Carbon and its Compounds
- Chapter 5 Periodic Classification of Elements
- Chapter 6 Life Processes
- Chapter 7 Control and Coordination
- Chapter 8 How do Organisms Reproduce
- Chapter 9 Heredity and Evolution
- Chapter 10 Light Reflection and Refraction
- Chapter 11 Human Eye and Colourful World
- Chapter 12 Electricity
- Chapter 13 Magnetic Effects of Electric Current
- Chapter 14 Sources of Energy
- Chapter 15 Our Environment
- Chapter 16 Management of Natural Resources
We hope the NCERT Exemplar Class 10 Science Chapter 2 Acids, Bases And Salts help you. If you have any query regarding NCERT Exemplar Class 10 Science Chapter 2 Acids, Bases And Salts, drop a comment below and we will get back to you at the earliest.
